- English
- 简体中文
- Español
- Português
- русский
- Français
- 日本語
- Deutsch
- tiếng Việt
- Italiano
- Nederlands
- ภาษาไทย
- Polski
- 한국어
- Svenska
- magyar
- Malay
- বাংলা ভাষার
- Dansk
- Suomi
- हिन्दी
- Pilipino
- Türkçe
- Gaeilge
- العربية
- Indonesia
- Norsk
- تمل
- český
- ελληνικά
- український
- Javanese
- فارسی
- தமிழ்
- తెలుగు
- नेपाली
- Burmese
- български
- ລາວ
- Latine
- Қазақша
- Euskal
- Azərbaycan
- Slovenský jazyk
- Македонски
- Lietuvos
- Eesti Keel
- Română
- Slovenski
- मराठी
- Srpski језик
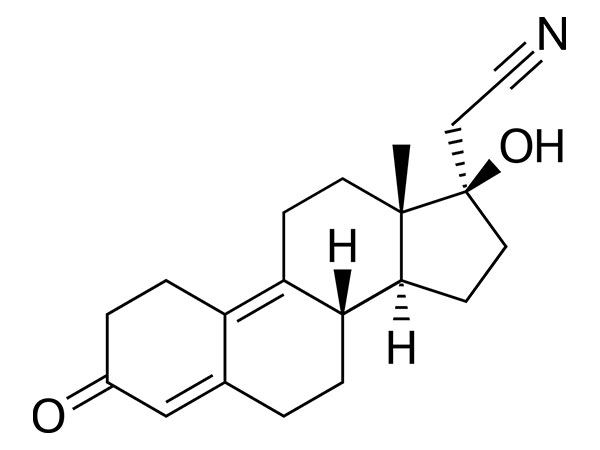
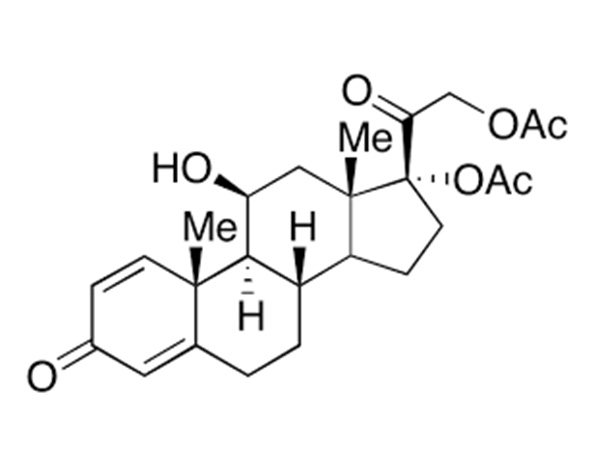
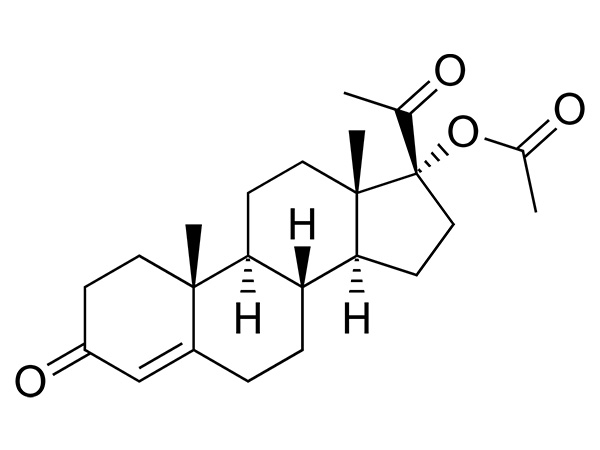
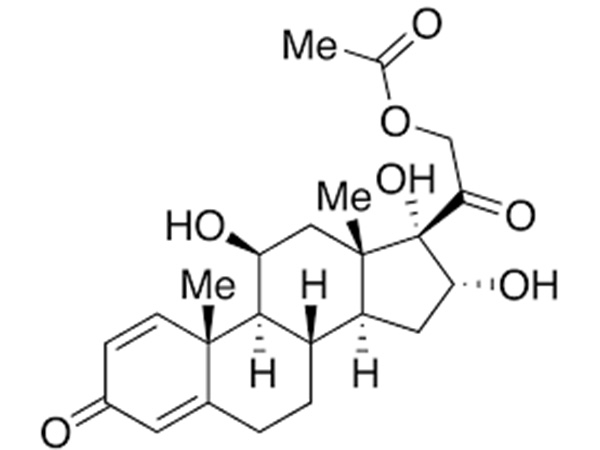
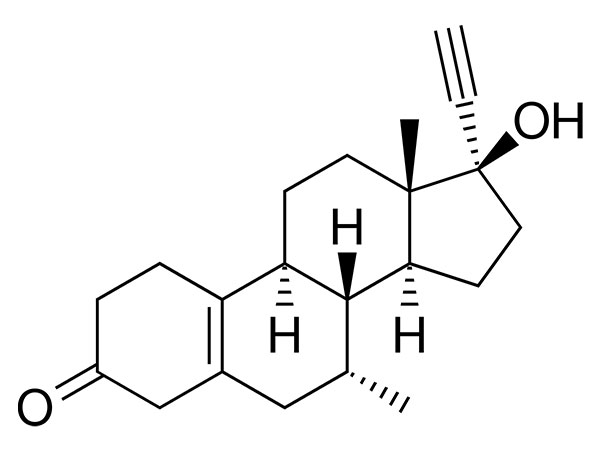
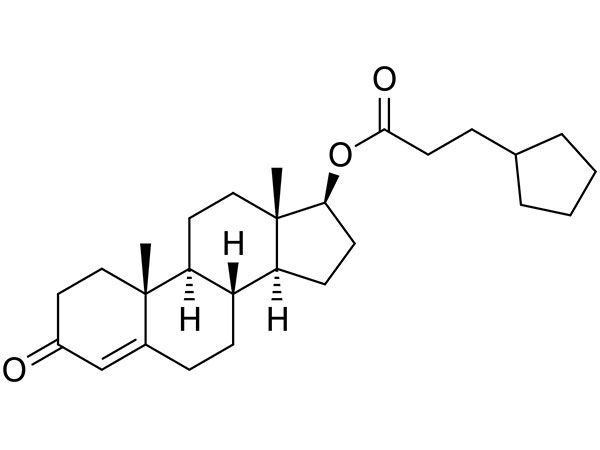
China CAS:65928-58-7 Manufacturer
We are going to make every single effort for being excellent and excellent, and accelerate our ways for standing while in the rank of international top-grade and high-tech enterprises for CAS:65928-58-7, We welcome clients, enterprise associations and mates from all parts in the world to speak to us and search for cooperation for mutual positive aspects.
CAS:65928-58-7, We take measure at any expense to achieve essentially the most up-to-date equipment and approaches. The packing of nominated brand is our a further distinguishing feature. The products to assure years of trouble-free service has attracted a great deal customers. The solutions are obtainable in improved designs and richer assortment, they're created scientifically of purely raw supplies. It readily available in a variety of designs and specs for your selection. The most recent kinds are a great deal better than the preceding one particular and they are quite popular with lots of prospects.
Hot Products
Methylprednisolone Hemisuccinate
For Methylprednisolone Hemisuccinate, we have EP, USP and CP specification. DMF and WC available.CAS:2921-57-5
Tibolone
Tibolone complies with EP specification. DMF is approved.CAS:5630-53-5
Testosterone Cypionate
Testosterone Cypionate has USP specification. TP available and DMF under filing.CAS:58-20-8
Valganciclovir Hydrochloride
Valganciclovir Hydrochloride has USP specification. DMF approved.CAS:175865-59-5
Oxcarbazepine
Oxcarbazepine has CP, EP and USP specifications. CEP and DMF available, GMP approved.CAS:28721-07-5
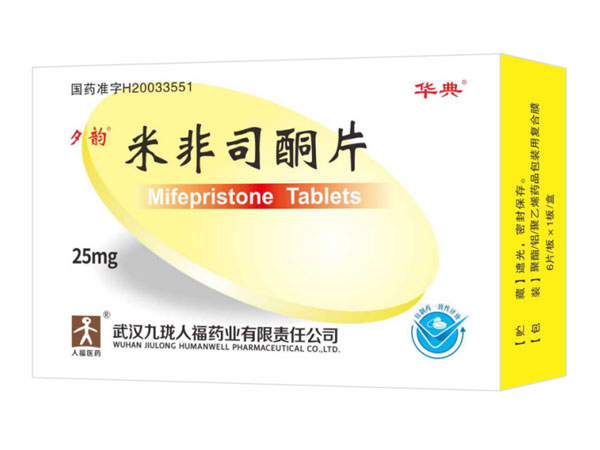
Mifepristone Tablets 25mg*6
Mifepristone Tablets 25mg*6
Indications:Abortion