- English
- 简体中文
- Español
- Português
- русский
- Français
- 日本語
- Deutsch
- tiếng Việt
- Italiano
- Nederlands
- ภาษาไทย
- Polski
- 한국어
- Svenska
- magyar
- Malay
- বাংলা ভাষার
- Dansk
- Suomi
- हिन्दी
- Pilipino
- Türkçe
- Gaeilge
- العربية
- Indonesia
- Norsk
- تمل
- český
- ελληνικά
- український
- Javanese
- فارسی
- தமிழ்
- తెలుగు
- नेपाली
- Burmese
- български
- ລາວ
- Latine
- Қазақша
- Euskal
- Azərbaycan
- Slovenský jazyk
- Македонски
- Lietuvos
- Eesti Keel
- Română
- Slovenski
- मराठी
- Srpski језик
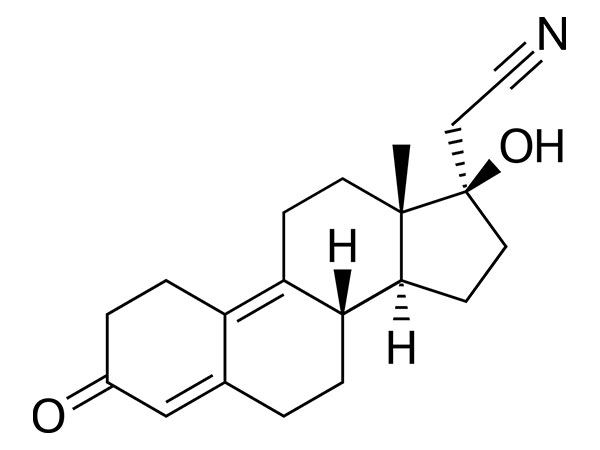
China CAS:65928-58-7 Manufacturer
We are going to make every single effort for being excellent and excellent, and accelerate our ways for standing while in the rank of international top-grade and high-tech enterprises for CAS:65928-58-7, We welcome clients, enterprise associations and mates from all parts in the world to speak to us and search for cooperation for mutual positive aspects.
CAS:65928-58-7, We take measure at any expense to achieve essentially the most up-to-date equipment and approaches. The packing of nominated brand is our a further distinguishing feature. The products to assure years of trouble-free service has attracted a great deal customers. The solutions are obtainable in improved designs and richer assortment, they're created scientifically of purely raw supplies. It readily available in a variety of designs and specs for your selection. The most recent kinds are a great deal better than the preceding one particular and they are quite popular with lots of prospects.
Hot Products
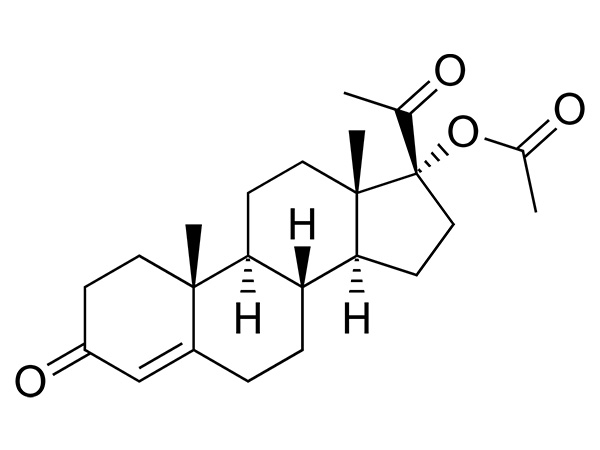
Medroxyprogesterone Acetate
Medroxyprogesterone Acetate has USP、EP、 IP、JP and KP. DMF, GMP is available.CAS:71-58-9
Nintedanib Esylate
Nintedanib Esylate has in-house specification. DMF available.CAS:656247-18-6
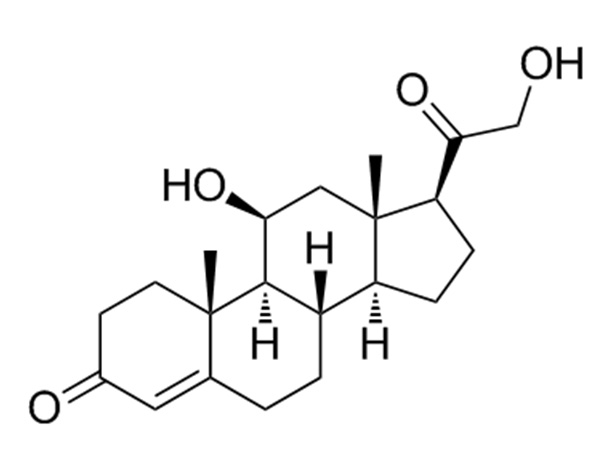
17a-Hydroxyprogesterone Acetate
17a-Hydroxyprogesterone Acetate is an endogenous progesteroid hormone similar to progesterone.CAS:302-23-8
Compound Mifepristone Tablets
Compound Mifepristone Tablets Specifications:Mifepristone 30mg Anorethidrane 5mg*2
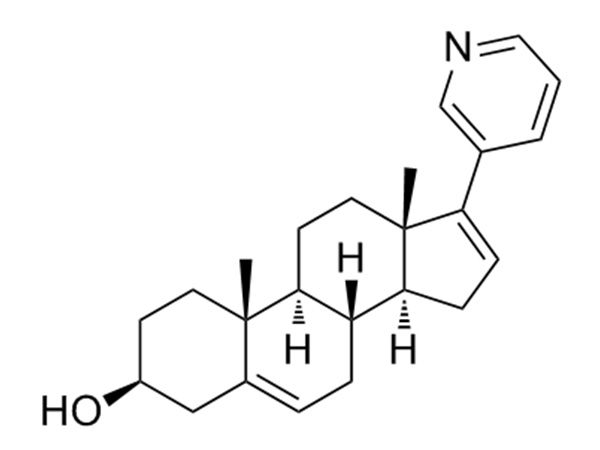
Indications:AbortionAbiraterone
Abiraterone is a steroidal cytochrome P 450 17α-hydroxylase-17,20-lyase inhibitor (CYP17), It is used in combination with prednisone to treat patients with metastatic castration-resistant prostate cancer (prostate cancer that is resistant to medical or surgical treatments that lower testosterone and has already spread to other parts of the body) and metastatic high-risk castration-sensitive prostate cancer.CAS:154229-19-3
3-Oxo-4-androsten-17β-carboxylic acid
3-Oxo-4-androsten-17β-carboxylic acid is an intermediate of Dutasteride.CAS:302-97-6