- English
- 简体中文
- Español
- Português
- русский
- Français
- 日本語
- Deutsch
- tiếng Việt
- Italiano
- Nederlands
- ภาษาไทย
- Polski
- 한국어
- Svenska
- magyar
- Malay
- বাংলা ভাষার
- Dansk
- Suomi
- हिन्दी
- Pilipino
- Türkçe
- Gaeilge
- العربية
- Indonesia
- Norsk
- تمل
- český
- ελληνικά
- український
- Javanese
- فارسی
- தமிழ்
- తెలుగు
- नेपाली
- Burmese
- български
- ລາວ
- Latine
- Қазақша
- Euskal
- Azərbaycan
- Slovenský jazyk
- Македонски
- Lietuvos
- Eesti Keel
- Română
- Slovenski
- मराठी
- Srpski језик
China Rafenacin Manufacturer
With this motto in mind, we've turn into one of quite possibly the most technologically innovative, cost-efficient, and price-competitive manufacturers for Rafenacin,CAS:864750-70-9, "Quality", "honesty" and "service" is our principle. Our loyalty and commitments remain respectfully at your support. Speak to Us Today For even more facts, get in touch with us now.
Rafenacin, We have constructed strong and long co-operation relationship with an enormous quantity of companies within this business overseas. Immediate and specialist after-sale service supplied by our consultant group has happy our buyers. Detailed Info and parameters from the merchandise will probably be sent to you for any thorough acknowledge. Free samples may be delivered and company check out to our corporation. n Portugal for negotiation is constantly welcome. Hope to get inquiries type you and construct a long-term co-operation partnership.
Hot Products
Methylprednisolone
Methylprednisolone has USP, EP, IP, JP and KP specifications. DMF and WC available.CAS:83-43-2
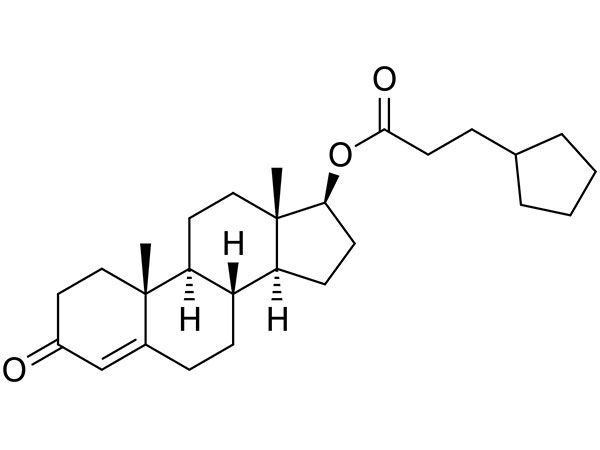
Testosterone Cypionate
Testosterone Cypionate has USP specification. TP available and DMF under filing.CAS:58-20-8
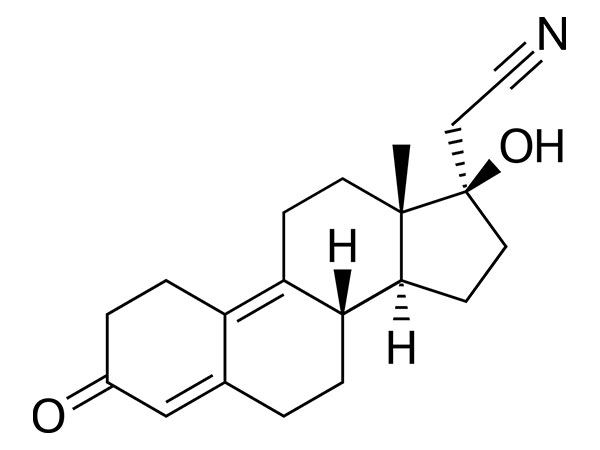
Dienogest
Dienogest has EP specifications. DMF approved.CAS:65928-58-7
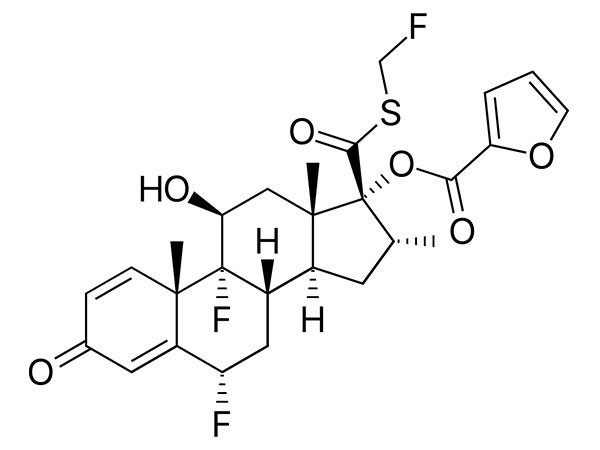
Fluticasone Furoate
Fluticasone furoate has In-house specifications. DMF under filing.CAS:397864-44-7
Compound Mifepristone Tablets
Compound Mifepristone Tablets Specifications:Mifepristone 30mg Anorethidrane 5mg*2
Indications:AbortionMisoprostol Tablets 0.2mg*3
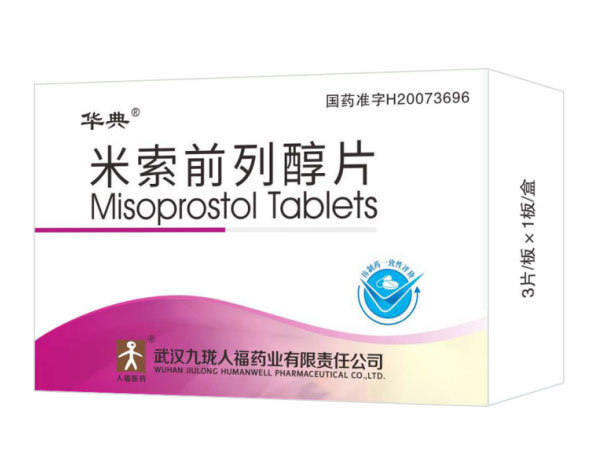
Misoprostol Tablets 0.2mg*3
Specifications:0.2mg*3
Indications:Abortion