- English
- 简体中文
- Español
- Português
- русский
- Français
- 日本語
- Deutsch
- tiếng Việt
- Italiano
- Nederlands
- ภาษาไทย
- Polski
- 한국어
- Svenska
- magyar
- Malay
- বাংলা ভাষার
- Dansk
- Suomi
- हिन्दी
- Pilipino
- Türkçe
- Gaeilge
- العربية
- Indonesia
- Norsk
- تمل
- český
- ελληνικά
- український
- Javanese
- فارسی
- தமிழ்
- తెలుగు
- नेपाली
- Burmese
- български
- ລາວ
- Latine
- Қазақша
- Euskal
- Azərbaycan
- Slovenský jazyk
- Македонски
- Lietuvos
- Eesti Keel
- Română
- Slovenski
- मराठी
- Srpski језик
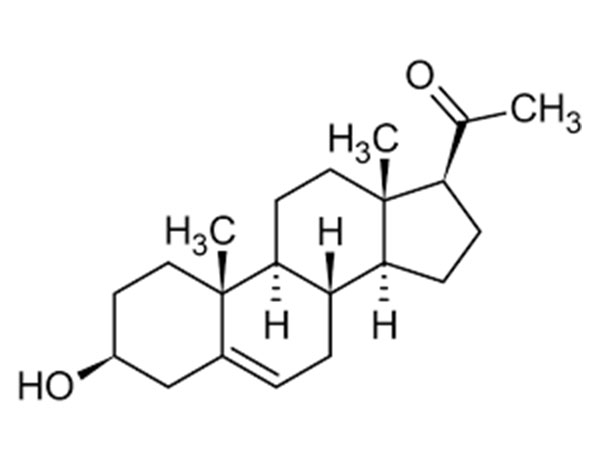
China Prasterone Acetate Manufacturer
Our primary goal is to offer our clients a serious and responsible business relationship, providing personalized attention to all of them for Prasterone Acetate,CAS:853-23-6,DHEA Acetate, Safety by innovation is our promise to each other.
Prasterone Acetate, Our expert engineering team will generally be prepared to serve you for consultation and feedback. We're able to also give you with free of charge samples to meet your requirements. Best efforts will likely be produced to provide you the best service and merchandise. When you are keen on our business and items, make sure you speak to us by sending us emails or call us quickly. In an effort to know our merchandise and company extra, you may come to our factory to view it. We'll generally welcome guests from all over the world to our business to create business relations with us. Be sure to feel cost-free to speak to us for small business and we believe we are going to share the best trading experience with all our merchants.
Hot Products
Ganciclovir
Ganciclovir has CP ,EP, USP specification. DMF and GMP approved.CAS:82410-32-0
Testosterone Undecanoate
Testosterone undecanoate has CP specification. TP available and DMF under filing.CAS:5949-44-0
Estriol
Estriol has CP, EP, USP specifications. DMF under filing.CAS:50-27-1
Oseltamivir Phosphate
Oseltamivir Phosphate has CP ,EP and USP specifications. DMF available.CAS:204255-11-8
Mirabegron
Mirabegron has In-house specification. DMF approved..CAS:223673-61-8
Cariprazine hydrochloride
Cariprazine hydrochloride has In-house specification. DMF approved.CAS:1083076-69-0