- English
- 简体中文
- Español
- Português
- русский
- Français
- 日本語
- Deutsch
- tiếng Việt
- Italiano
- Nederlands
- ภาษาไทย
- Polski
- 한국어
- Svenska
- magyar
- Malay
- বাংলা ভাষার
- Dansk
- Suomi
- हिन्दी
- Pilipino
- Türkçe
- Gaeilge
- العربية
- Indonesia
- Norsk
- تمل
- český
- ελληνικά
- український
- Javanese
- فارسی
- தமிழ்
- తెలుగు
- नेपाली
- Burmese
- български
- ລາວ
- Latine
- Қазақша
- Euskal
- Azərbaycan
- Slovenský jazyk
- Македонски
- Lietuvos
- Eesti Keel
- Română
- Slovenski
- मराठी
- Srpski језик
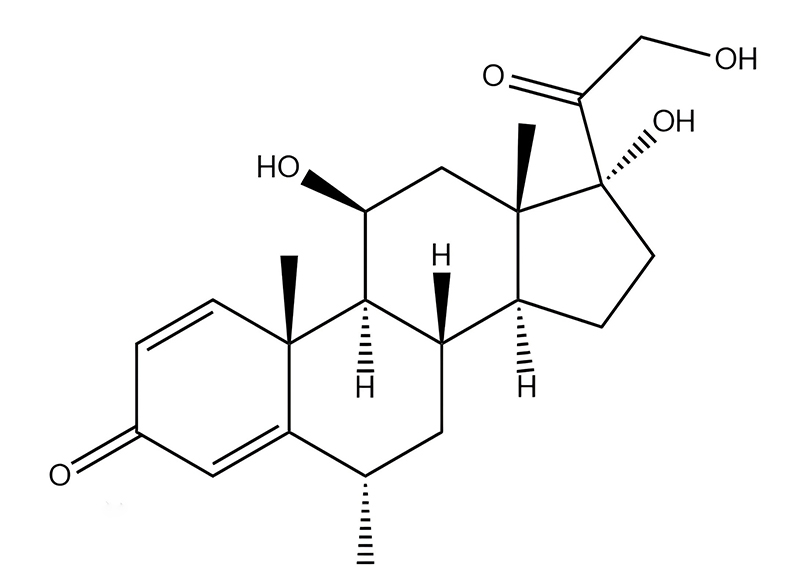
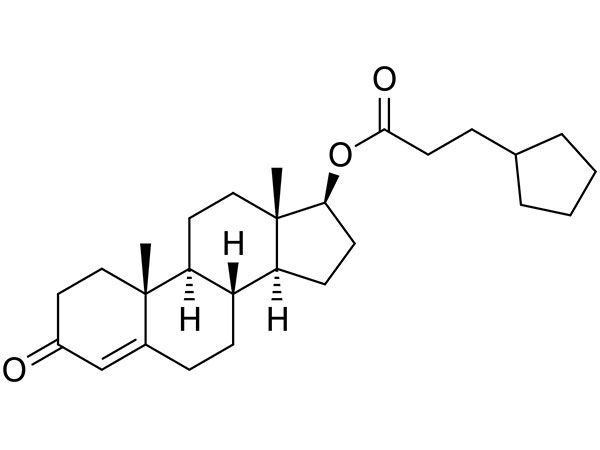
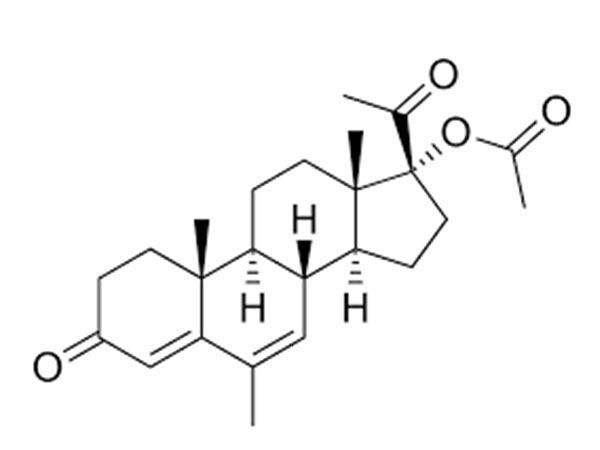
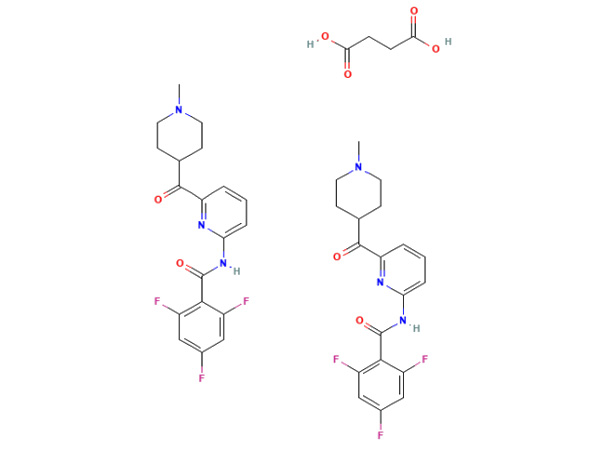
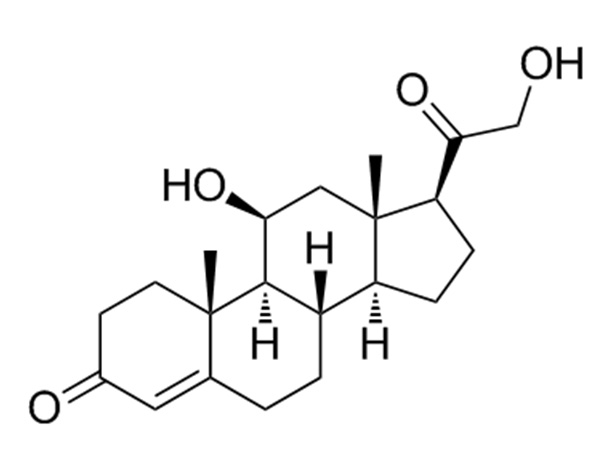
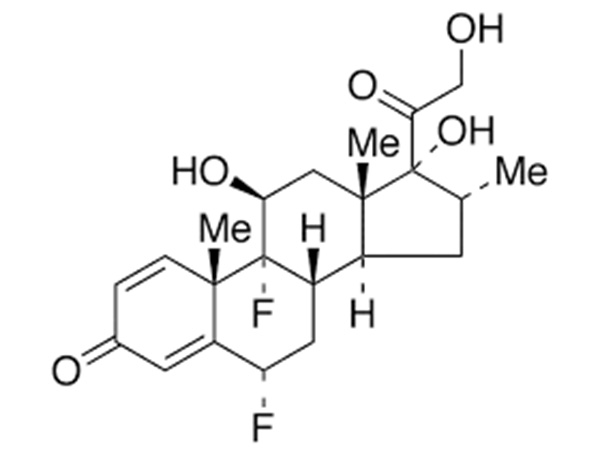
China CAS:51333-22-3 Manufacturer
Our primary intention should be to offer our clientele a serious and responsible enterprise relationship, delivering personalized attention to all of them for CAS:51333-22-3, We're seeking forward to forming successful business enterprise romantic relationship with new shoppers in the around upcoming!
CAS:51333-22-3, With its rich manufacturing experience, high-quality products, and perfect after-sale service, the company has gained good reputation and has become one of the famous enterprise specialized in manufacturing series.We sincerely hope to establish business relation with you and pursue mutual benefit.
Hot Products
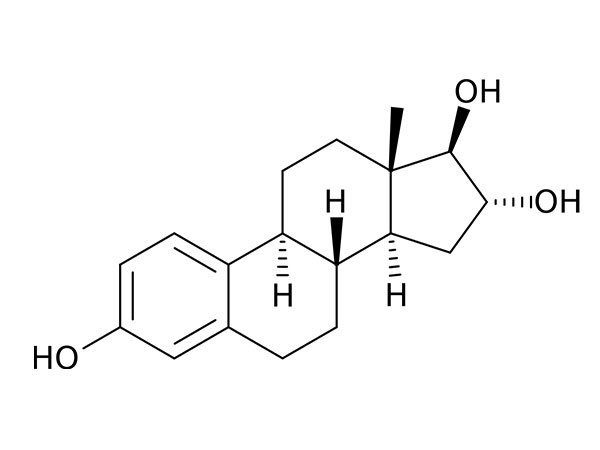
Estriol
Estriol has CP, EP, USP specifications. DMF under filing.CAS:50-27-1
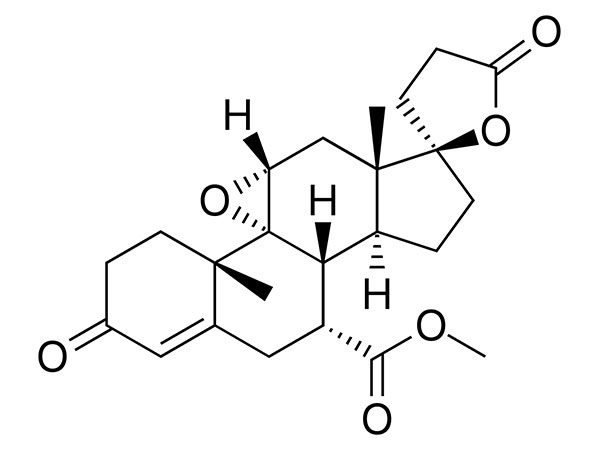
Eplerenone
Eplerenone has EP specifications. CEP available and FDA approved.CAS:107724-20-9
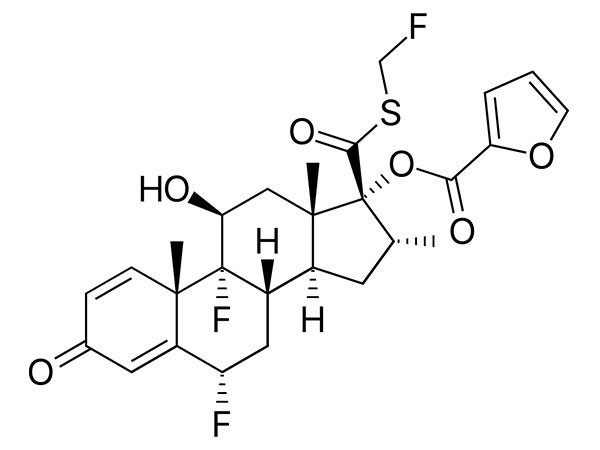
Fluticasone Furoate
Fluticasone furoate has In-house specifications. DMF under filing.CAS:397864-44-7
Mifepristone Capsules
Mifepristone Capsules Specifications:10mg*1
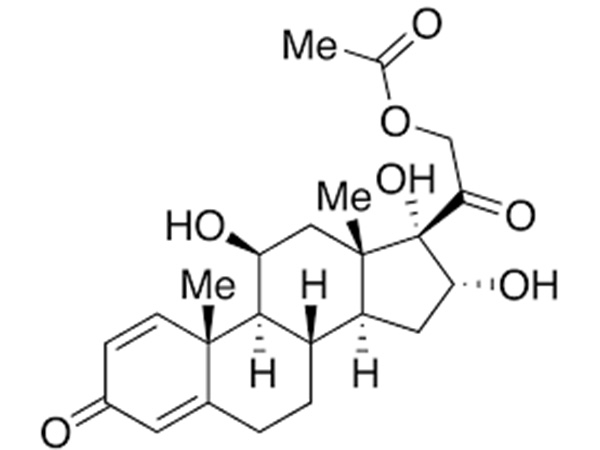
Indications:Emergency Contraceptive3-Oxo-4-androsten-17β-carboxylic acid
3-Oxo-4-androsten-17β-carboxylic acid is an intermediate of Dutasteride.CAS:302-97-6
Flumethasone
Flumethasone is a glucocorticoid, an anti-inflammatory. It can promptly decrease inflammation, exudation and itching is experienced after application.CAS:2135-17-3