- English
- 简体中文
- Español
- Português
- русский
- Français
- 日本語
- Deutsch
- tiếng Việt
- Italiano
- Nederlands
- ภาษาไทย
- Polski
- 한국어
- Svenska
- magyar
- Malay
- বাংলা ভাষার
- Dansk
- Suomi
- हिन्दी
- Pilipino
- Türkçe
- Gaeilge
- العربية
- Indonesia
- Norsk
- تمل
- český
- ελληνικά
- український
- Javanese
- فارسی
- தமிழ்
- తెలుగు
- नेपाली
- Burmese
- български
- ລາວ
- Latine
- Қазақша
- Euskal
- Azərbaycan
- Slovenský jazyk
- Македонски
- Lietuvos
- Eesti Keel
- Română
- Slovenski
- मराठी
- Srpski језик
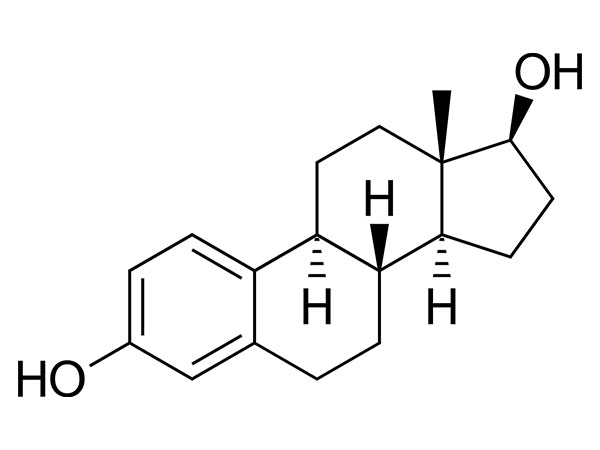
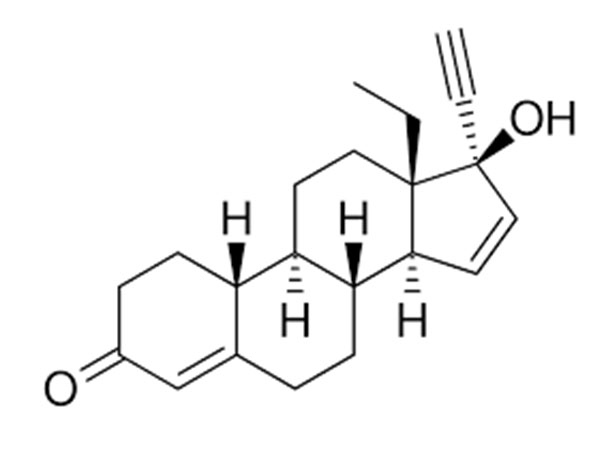
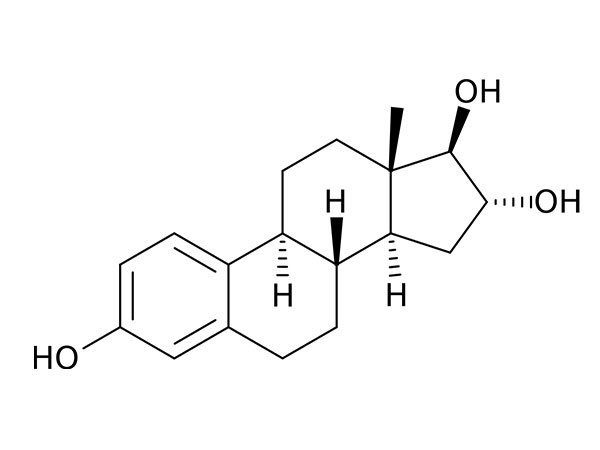
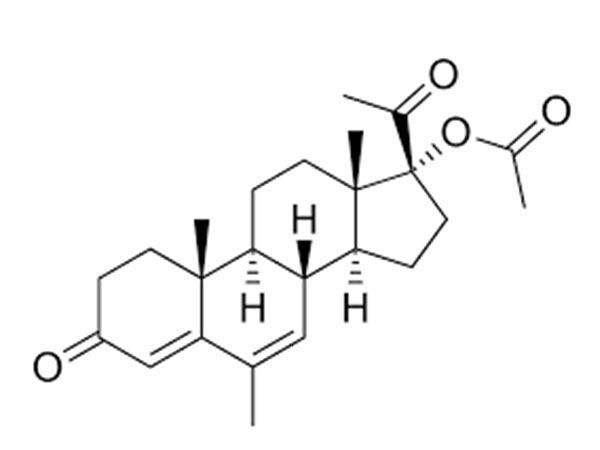
China CAS:35380-71-3 Manufacturer
We hold strengthening and perfecting our items and repair. At the same time, we get the job done actively to do research and progress for CAS:35380-71-3, We welcome new and aged buyers from all walks of lifetime to make contact with us for potential small business associations and mutual success!
CAS:35380-71-3, Our company warmly invites domestic and overseas customers to come and negotiate business with us. Let us join hands to create a brilliant tomorrow! We're looking forward to cooperating with you sincerely to achieve a win-win situation. We promise to try our best to supply you with high quality and efficient services.
Hot Products
Tibolone
Tibolone complies with EP specification. DMF is approved.CAS:5630-53-5
Gestodene
Gestodene has CP and EP specification. DMF, GMP available.CAS:60282-87-3
Estradiol Valerate
Estradiol Valerate has CP specification. DMF approved.CAS:979-32-8
Mirabegron
Mirabegron has In-house specification. DMF approved..CAS:223673-61-8
DHEA Acetate(Prasterone Acetate)
DHEA Acetate(Prasterone Acetate) is the intermediate of DHEA(prasterone).CAS:853-23-6
Cyproterone Acetate Tablets
Cyproterone Acetate Tablets Specifications:50mg*24
Indications:Anti-androgen