- English
- 简体中文
- Español
- Português
- русский
- Français
- 日本語
- Deutsch
- tiếng Việt
- Italiano
- Nederlands
- ภาษาไทย
- Polski
- 한국어
- Svenska
- magyar
- Malay
- বাংলা ভাষার
- Dansk
- Suomi
- हिन्दी
- Pilipino
- Türkçe
- Gaeilge
- العربية
- Indonesia
- Norsk
- تمل
- český
- ελληνικά
- український
- Javanese
- فارسی
- தமிழ்
- తెలుగు
- नेपाली
- Burmese
- български
- ລາວ
- Latine
- Қазақша
- Euskal
- Azərbaycan
- Slovenský jazyk
- Македонски
- Lietuvos
- Eesti Keel
- Română
- Slovenski
- मराठी
- Srpski језик
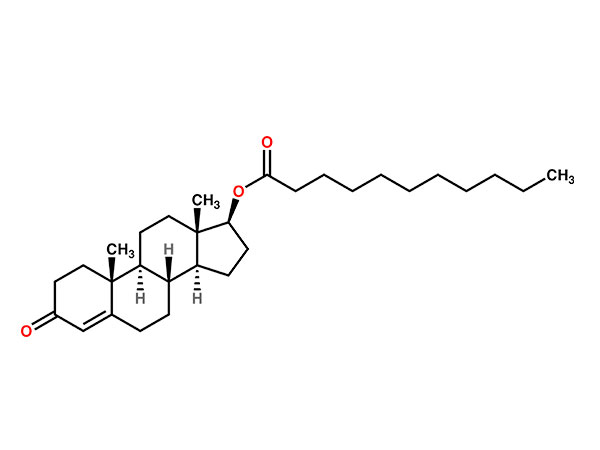
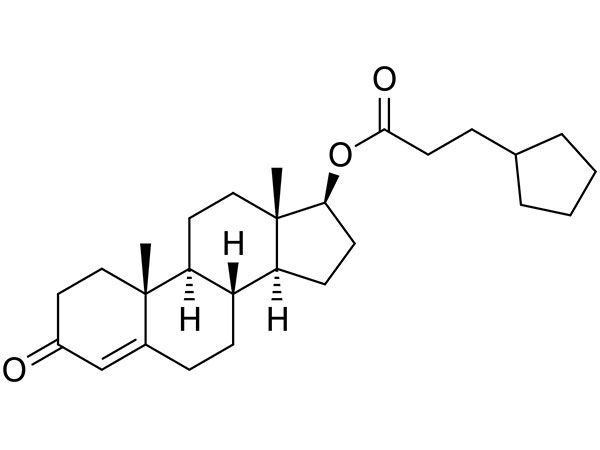
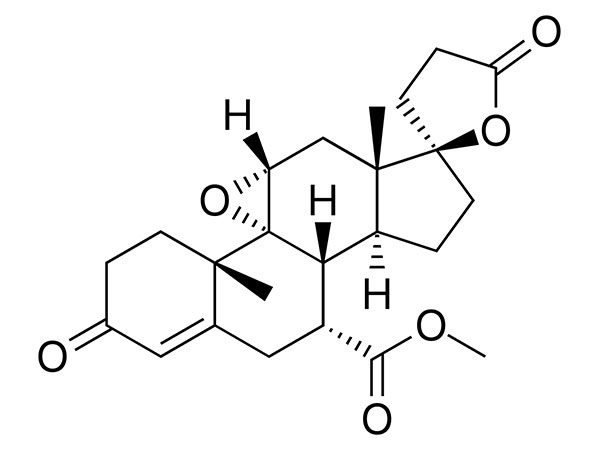
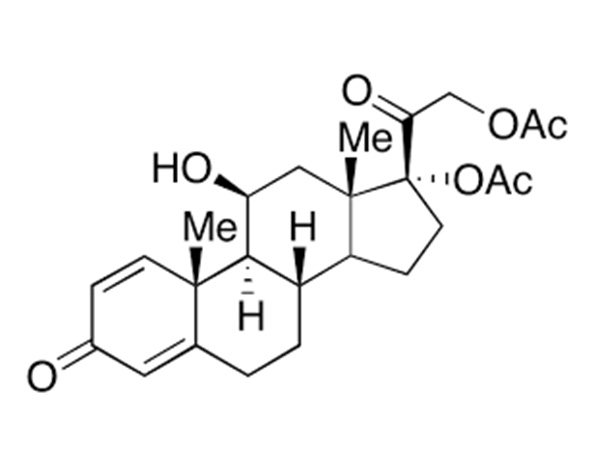
China CAS:58-22-0 Manufacturer
To be a result of ours specialty and repair consciousness, our corporation has won a good popularity amid consumers everywhere in the environment for CAS:58-22-0, We warmly welcome prospects, organization associations and mates from everywhere in the earth to get in touch with us and request cooperation for mutual benefits.
CAS:58-22-0, With high quality, reasonable price, on-time delivery and customized & customized services to help customers achieve their goals successfully, our company has got praise in both domestic and foreign markets. Buyers are welcome to contact us.
Hot Products
Desonide
Desonide has USP and EP specifications. DMF available.CAS:638-94-8
Testosterone Cypionate
Testosterone Cypionate has USP specification. TP available and DMF under filing.CAS:58-20-8
Eplerenone
Eplerenone has EP specifications. CEP available and FDA approved.CAS:107724-20-9
Oseltamivir Phosphate
Oseltamivir Phosphate has CP ,EP and USP specifications. DMF available.CAS:204255-11-8
21-acetoxy-11β-hydroxypregna-1,4,16-triene-3,20-dione
21-Acetoxy-11β-hydroxypregna-1,4,16-triene-3,20-dione is an intermediate in the synthesis of Budesonide (B689490) related derivatives.CAS:3044-42-6
Cariprazine hydrochloride
Cariprazine hydrochloride has In-house specification. DMF approved.CAS:1083076-69-0