- English
- 简体中文
- Español
- Português
- русский
- Français
- 日本語
- Deutsch
- tiếng Việt
- Italiano
- Nederlands
- ภาษาไทย
- Polski
- 한국어
- Svenska
- magyar
- Malay
- বাংলা ভাষার
- Dansk
- Suomi
- हिन्दी
- Pilipino
- Türkçe
- Gaeilge
- العربية
- Indonesia
- Norsk
- تمل
- český
- ελληνικά
- український
- Javanese
- فارسی
- தமிழ்
- తెలుగు
- नेपाली
- Burmese
- български
- ລາວ
- Latine
- Қазақша
- Euskal
- Azərbaycan
- Slovenský jazyk
- Македонски
- Lietuvos
- Eesti Keel
- Română
- Slovenski
- मराठी
- Srpski језик
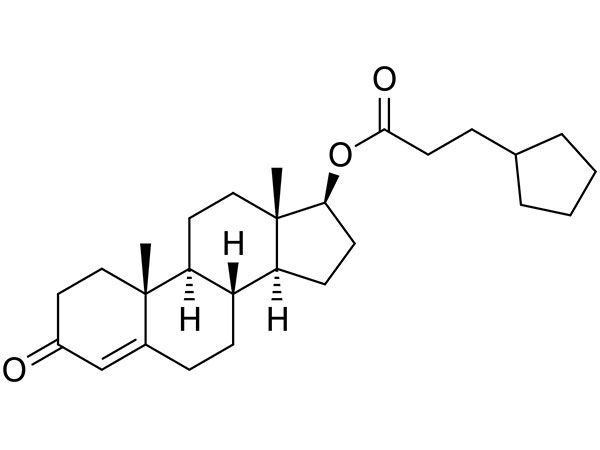
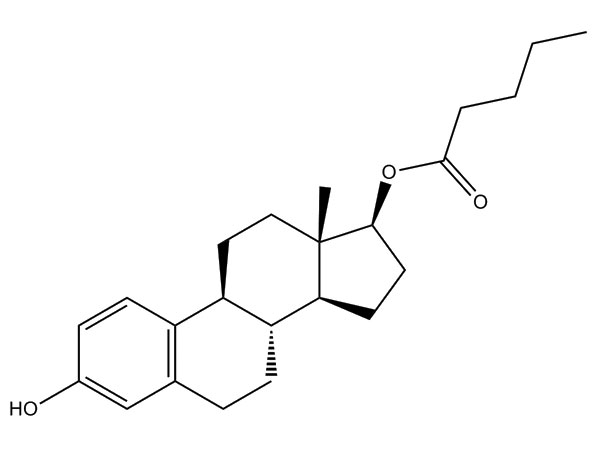
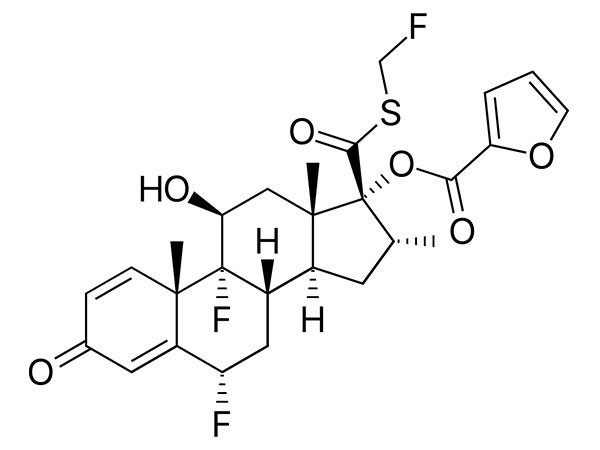
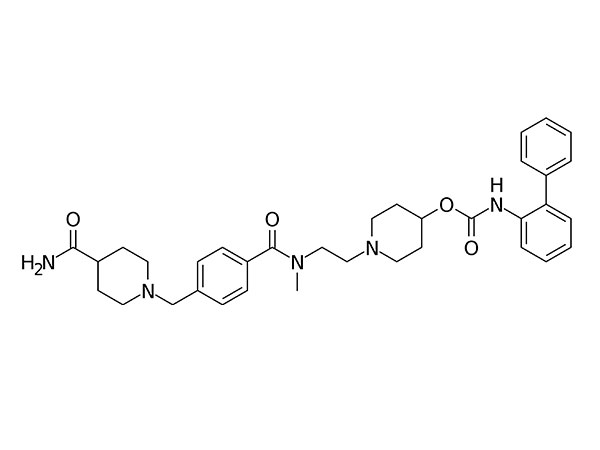
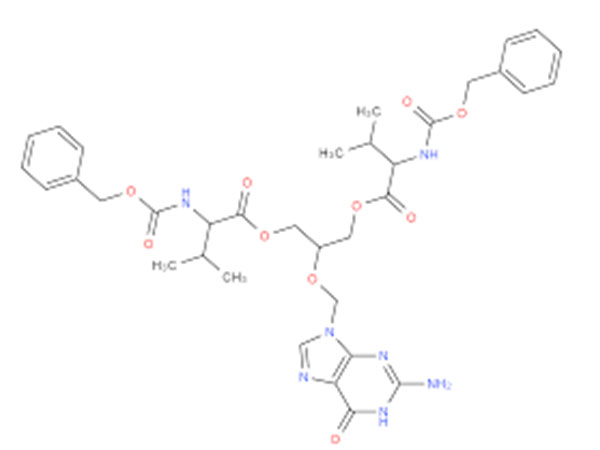
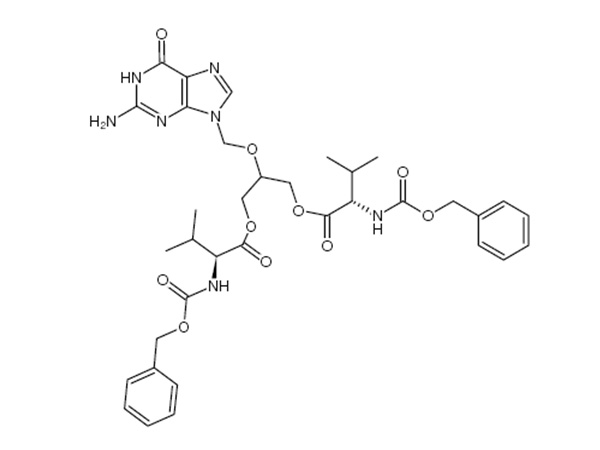
China CAS:28721-07-5 Manufacturer
We not only will try our greatest to offer superb companies to just about every buyer, but also are ready to receive any suggestion offered by our shoppers for CAS:28721-07-5, We are going to do our best to fulfill your specifications and are sincerely searching forward to building mutual valuable organization romantic relationship along with you!
CAS:28721-07-5, Welcome to visit our company and factory, there are various solutions displayed in our showroom that will meet your expectation, meanwhile, if you are convenient to visit our website, our sales staff will try their efforts to deliver you the best service
Hot Products
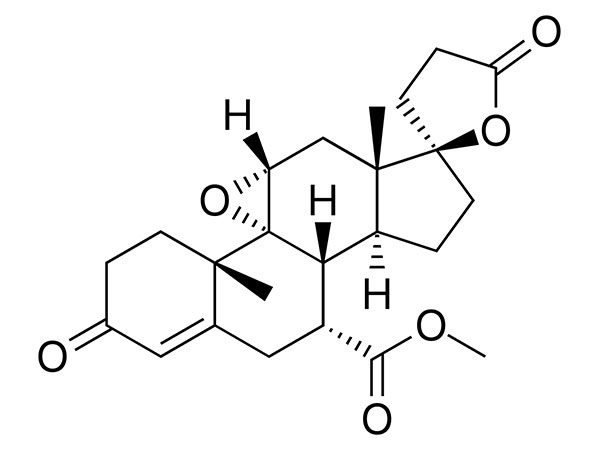
Eplerenone
Eplerenone has EP specifications. CEP available and FDA approved.CAS:107724-20-9
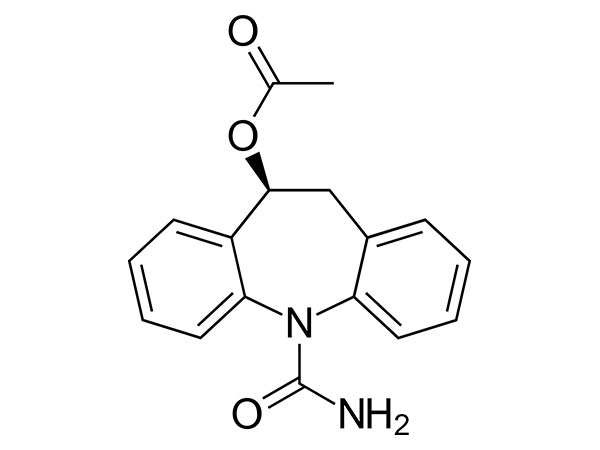
Eslicarbazepine Acetate
Eslicarbazepine Acetate has In-house specification. DMF approved.CAS:236395-14-5
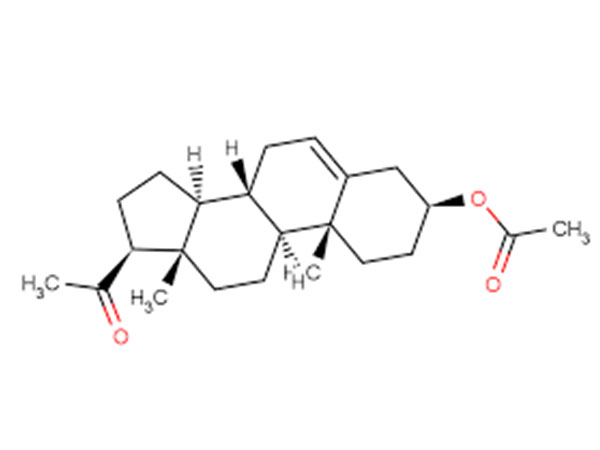
Pregnenolone Acetate
Pregnenolone AcetateCAS:1778-02-5
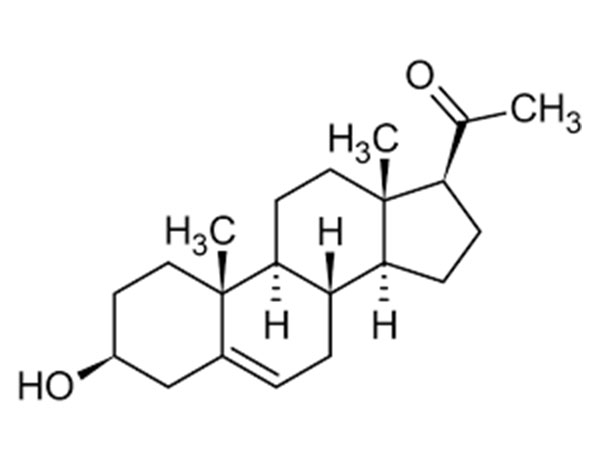
Pregnenolone
PregnenoloneCAS:145-13-1
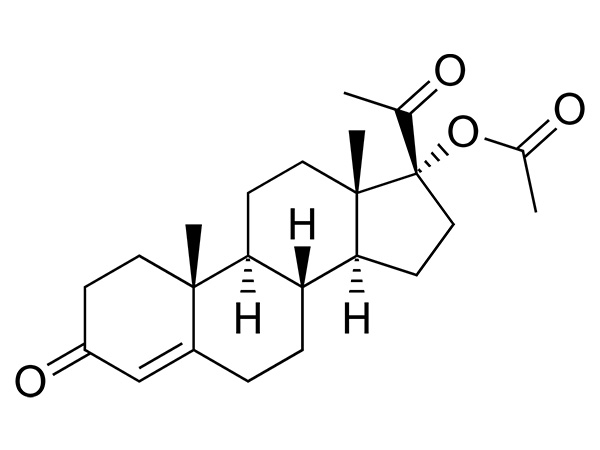
17a-Hydroxyprogesterone Acetate
17a-Hydroxyprogesterone Acetate is an endogenous progesteroid hormone similar to progesterone.CAS:302-23-8
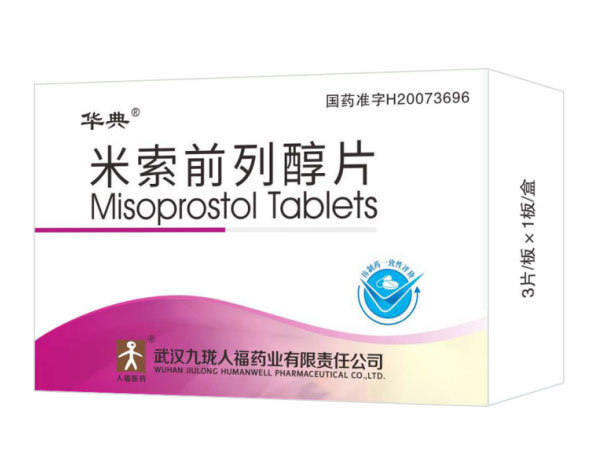
Misoprostol Tablets 0.2mg*3
Misoprostol Tablets 0.2mg*3
Specifications:0.2mg*3
Indications:Abortion