- English
- 简体中文
- Español
- Português
- русский
- Français
- 日本語
- Deutsch
- tiếng Việt
- Italiano
- Nederlands
- ภาษาไทย
- Polski
- 한국어
- Svenska
- magyar
- Malay
- বাংলা ভাষার
- Dansk
- Suomi
- हिन्दी
- Pilipino
- Türkçe
- Gaeilge
- العربية
- Indonesia
- Norsk
- تمل
- český
- ελληνικά
- український
- Javanese
- فارسی
- தமிழ்
- తెలుగు
- नेपाली
- Burmese
- български
- ລາວ
- Latine
- Қазақша
- Euskal
- Azərbaycan
- Slovenský jazyk
- Македонски
- Lietuvos
- Eesti Keel
- Română
- Slovenski
- मराठी
- Srpski језик
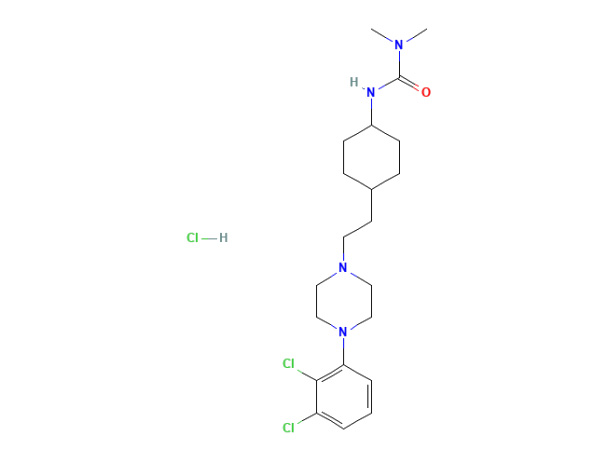
China CAS:1083076-69-0 Manufacturer
We insist over the principle of enhancement of 'High high quality, Efficiency, Sincerity and Down-to-earth working approach' to offer you with superb assistance of processing for CAS:1083076-69-0, We have exported to more than 40 countries and regions, which have gained fantastic popularity from our costumers everywhere in the globe.
CAS:1083076-69-0, We give good quality but unbeatable low price and the best service. Welcome to post your samples and color ring to us .We are going to produce the items according to your request. If you are interested in any solutions we provide, remember to feel free to contact us directly by mail, fax, telephone or internet. We have been here to answer your questions from Monday to Saturday and looking forward to cooperating with you.
Hot Products
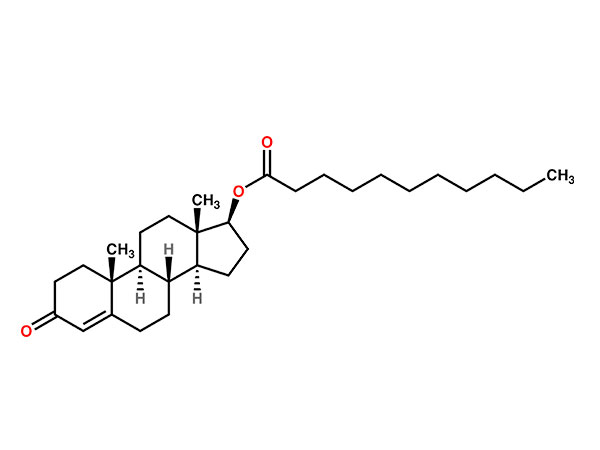
Abiraterone Acetate
Abiraterone Acetate has CP,USP specifications. DMF, WC available.CAS:154229-18-2
Nintedanib Esylate
Nintedanib Esylate has in-house specification. DMF available.CAS:656247-18-6
Mometasone Furoate
Mometasone furoate has CP、EP、USP specifications. DMF under filing.CAS:83919-23-7
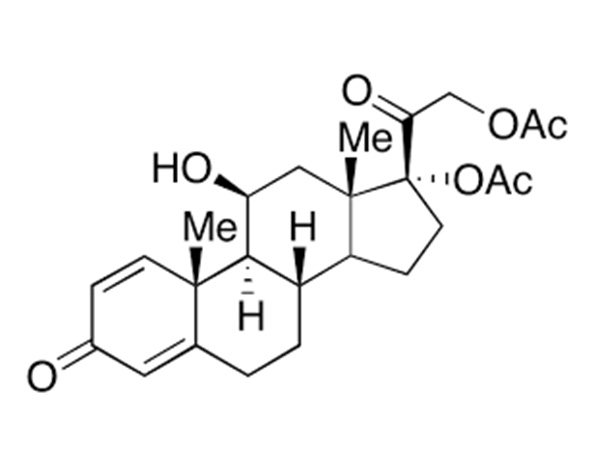
21-acetoxy-11β-hydroxypregna-1,4,16-triene-3,20-dione
21-Acetoxy-11β-hydroxypregna-1,4,16-triene-3,20-dione is an intermediate in the synthesis of Budesonide (B689490) related derivatives.CAS:3044-42-6
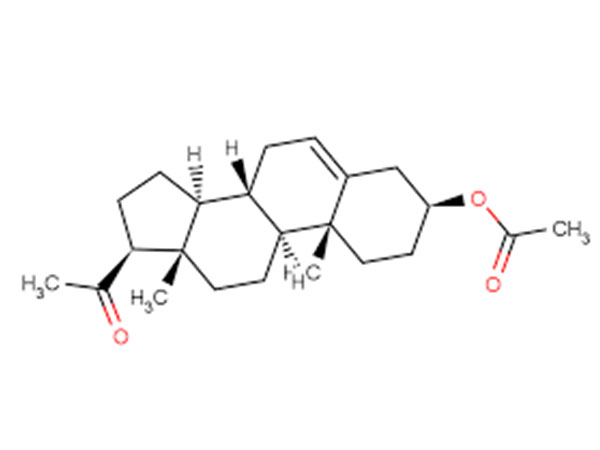
DHEA Acetate(Prasterone Acetate)
DHEA Acetate(Prasterone Acetate) is the intermediate of DHEA(prasterone).CAS:853-23-6
17a-Hydroxyprogesterone Acetate
17a-Hydroxyprogesterone Acetate is an endogenous progesteroid hormone similar to progesterone.CAS:302-23-8