- English
- 简体中文
- Español
- Português
- русский
- Français
- 日本語
- Deutsch
- tiếng Việt
- Italiano
- Nederlands
- ภาษาไทย
- Polski
- 한국어
- Svenska
- magyar
- Malay
- বাংলা ভাষার
- Dansk
- Suomi
- हिन्दी
- Pilipino
- Türkçe
- Gaeilge
- العربية
- Indonesia
- Norsk
- تمل
- český
- ελληνικά
- український
- Javanese
- فارسی
- தமிழ்
- తెలుగు
- नेपाली
- Burmese
- български
- ລາວ
- Latine
- Қазақша
- Euskal
- Azərbaycan
- Slovenský jazyk
- Македонски
- Lietuvos
- Eesti Keel
- Română
- Slovenski
- मराठी
- Srpski језик
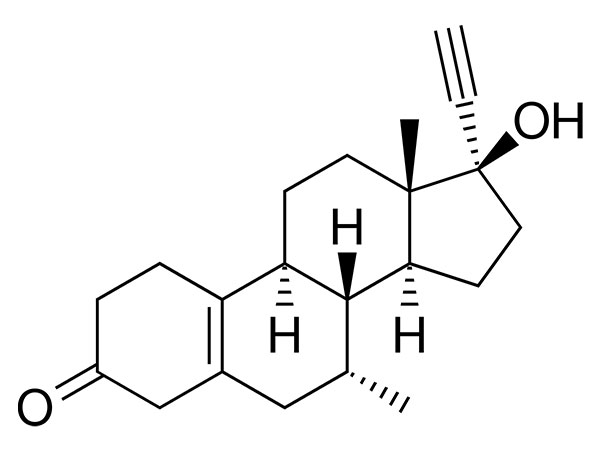
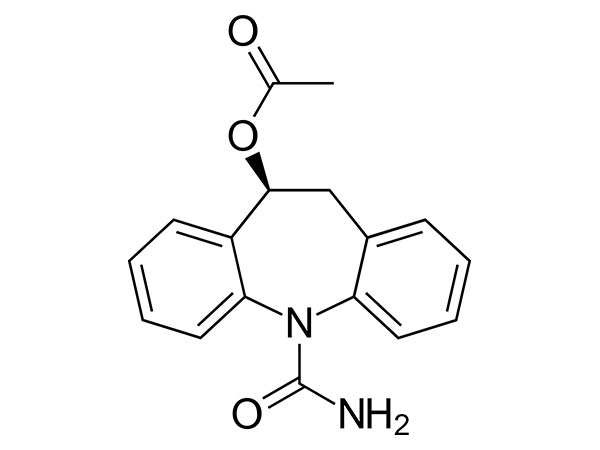
China CAS:187164-19-8 Manufacturer
We believe that prolonged time period partnership is really a result of top of the range, benefit added provider, prosperous knowledge and personal contact for CAS:187164-19-8, we could solve our customer problems asap and do the profit for our customer. For those who need superior provider and excellent , pls choose us , thanks !
CAS:187164-19-8, We have constructed strong and long co-operation relationship with an enormous quantity of companies within this business overseas. Immediate and specialist after-sale service supplied by our consultant group has happy our buyers. Detailed Info and parameters from the merchandise will probably be sent to you for any thorough acknowledge. Free samples may be delivered and company check out to our corporation. n Portugal for negotiation is constantly welcome. Hope to get inquiries type you and construct a long-term co-operation partnership.
Hot Products
Cyproterone Acetate
For Cyproterone Acetate (CPA), we have CP and EP specifications, CEP/TGA/EU-GMP available.CAS:427-51-0
Eslicarbazepine Acetate
Eslicarbazepine Acetate has In-house specification. DMF approved.CAS:236395-14-5
Revefenacin
Revefenacin has In-house specification. DMF approved.CAS:864750-70-9
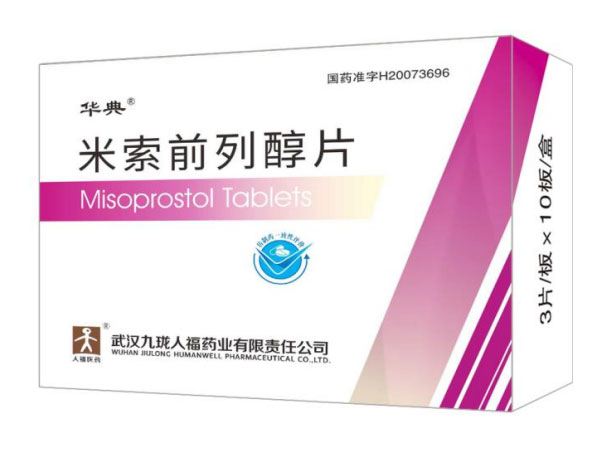
Misoprostol Tablets 0.2mg*30
Misoprostol Tablets 0.2mg*30
Indications:Gastric ulcerAbiraterone
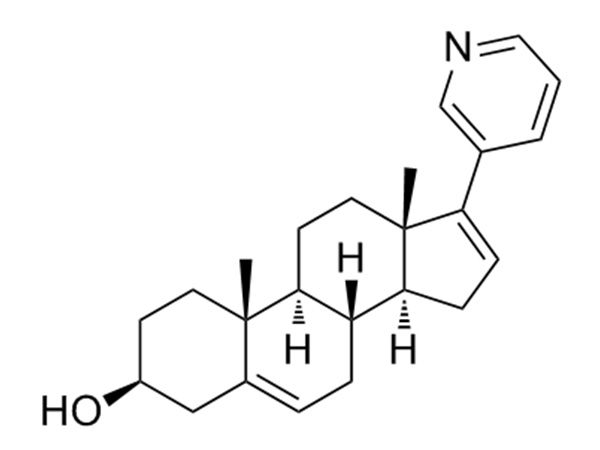
Abiraterone is a steroidal cytochrome P 450 17α-hydroxylase-17,20-lyase inhibitor (CYP17), It is used in combination with prednisone to treat patients with metastatic castration-resistant prostate cancer (prostate cancer that is resistant to medical or surgical treatments that lower testosterone and has already spread to other parts of the body) and metastatic high-risk castration-sensitive prostate cancer.CAS:154229-19-3
4-aza-5α-androstan-3-oxo-17β-carboxylic acid
4-aza-5α-androstan-3-oxo-17β-carboxylic acid is an intermediate of Dutasteride.CAS:104239-97-6