- English
- 简体中文
- Español
- Português
- русский
- Français
- 日本語
- Deutsch
- tiếng Việt
- Italiano
- Nederlands
- ภาษาไทย
- Polski
- 한국어
- Svenska
- magyar
- Malay
- বাংলা ভাষার
- Dansk
- Suomi
- हिन्दी
- Pilipino
- Türkçe
- Gaeilge
- العربية
- Indonesia
- Norsk
- تمل
- český
- ελληνικά
- український
- Javanese
- فارسی
- தமிழ்
- తెలుగు
- नेपाली
- Burmese
- български
- ລາວ
- Latine
- Қазақша
- Euskal
- Azərbaycan
- Slovenský jazyk
- Македонски
- Lietuvos
- Eesti Keel
- Română
- Slovenski
- मराठी
- Srpski језик
China CAS:107724-20-9 Manufacturer
We've been experienced manufacturer. Wining the majority on the crucial certifications of its market for CAS:107724-20-9, Top quality and competitive rates make our products and solutions appreciate a higher name all around the word.
CAS:107724-20-9, Our products are very popular in the word, like South American, Africa, Asia and so on. Companies to "create first-class products" as the goal, and strive to provide customers with high quality products, provide high-quality after-sales service and technical support, and customer mutual benefit, create a better career and future!
Hot Products
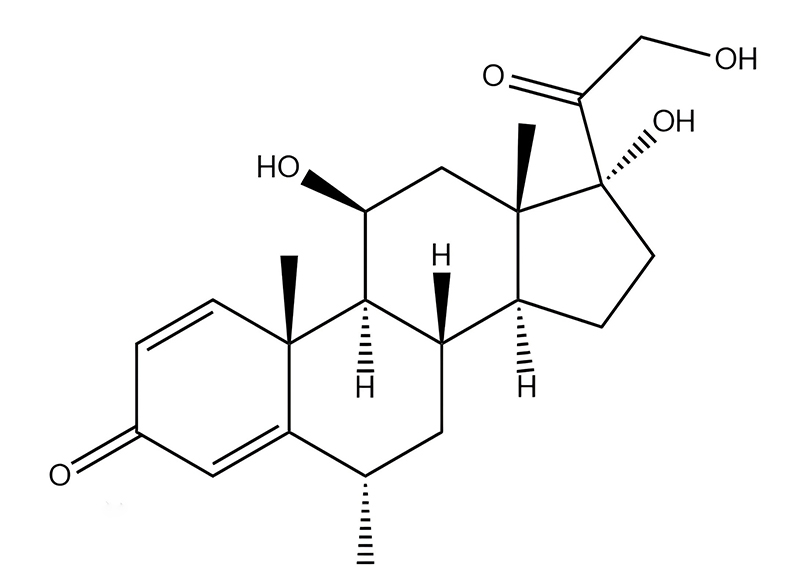
Methylprednisolone
Methylprednisolone has USP, EP, IP, JP and KP specifications. DMF and WC available.CAS:83-43-2
Medroxyprogesterone Acetate
Medroxyprogesterone Acetate has USP、EP、 IP、JP and KP. DMF, GMP is available.CAS:71-58-9
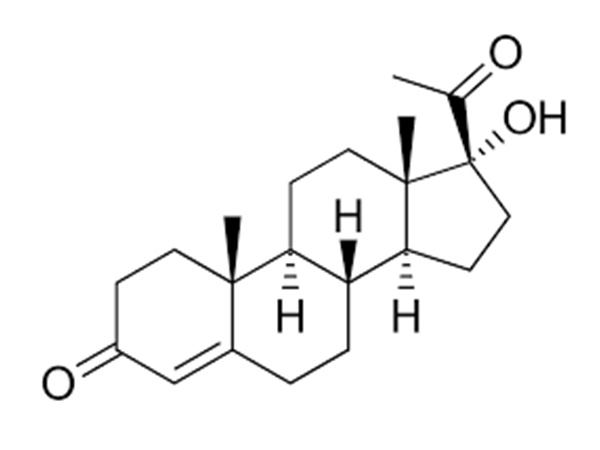
16-Dehydropregnenolone Acetate (16-DPA)
16-Dehydropregnenolone Acetate (16-DPA) is the dehydration product of Pregnenolone Acetate..CAS:979-02-2
17a-Hydroxyprogesterone
17a-Hydroxyprogesterone is an endogenous progesteroid hormone similar to progesterone.CAS:68-96-2
Misoprostol Tablets 0.2mg*30
Misoprostol Tablets 0.2mg*30
Indications:Gastric ulcerCompound Mifepristone Tablets
Compound Mifepristone Tablets Specifications:Mifepristone 30mg Anorethidrane 5mg*2
Indications:Abortion