- English
- 简体中文
- Español
- Português
- русский
- Français
- 日本語
- Deutsch
- tiếng Việt
- Italiano
- Nederlands
- ภาษาไทย
- Polski
- 한국어
- Svenska
- magyar
- Malay
- বাংলা ভাষার
- Dansk
- Suomi
- हिन्दी
- Pilipino
- Türkçe
- Gaeilge
- العربية
- Indonesia
- Norsk
- تمل
- český
- ελληνικά
- український
- Javanese
- فارسی
- தமிழ்
- తెలుగు
- नेपाली
- Burmese
- български
- ລາວ
- Latine
- Қазақша
- Euskal
- Azərbaycan
- Slovenský jazyk
- Македонски
- Lietuvos
- Eesti Keel
- Română
- Slovenski
- मराठी
- Srpski језик
China APIs Category Manufacturer
- View as
Estradiol Valerate
Estradiol Valerate has CP specification. DMF approved.
CAS:979-32-8
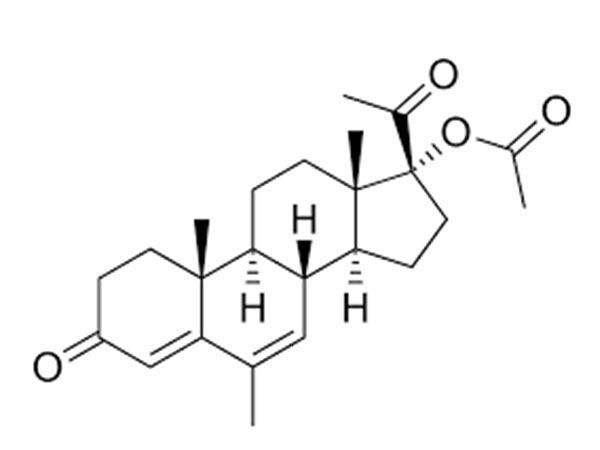
Read MoreSend InquiryMegestrol Acetate
Megestrol acetate has CP, EP and USP specifications. DMF under filing.
CAS:595-33-5
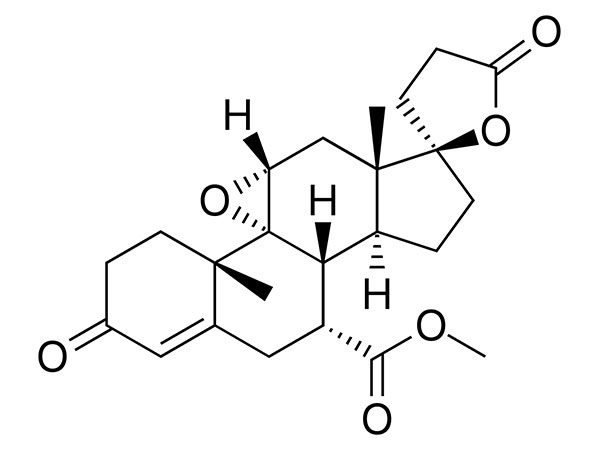
Read MoreSend InquiryEplerenone
Eplerenone has EP specifications. CEP available and FDA approved.
CAS:107724-20-9
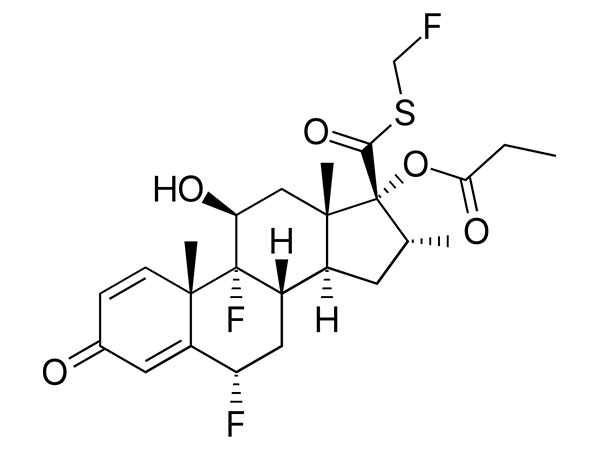
Read MoreSend InquiryFluticasone Propionate
Fluticasone propionate has CP, EP and USP specifications. DMF available.
CAS:80474-14-2
Read MoreSend Inquiry
Humanwell Pharmaceutical is one of the largest API manufacture in China. With more than 20 years of experience, we develop, manufacture and trade steroid APIs, intermediates and formulations. Our market covers all over the world, we have strong presences in North America, Europe, South America and Africa, with products sold to more than 150 countries.